Page 216 - Praxair - Specialty Gases and Equipment Reference Guide
P. 216
Laboratories
Analytical Instrumentation
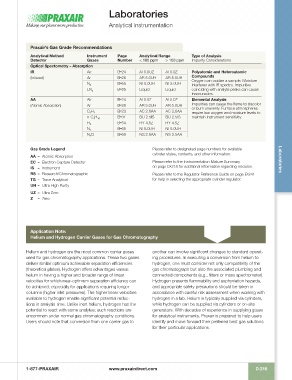
Praxair’s Gas Grade Recommendations
Analytical Method Instrument Page Analytical Range Type of Analysis
Detector Gases Number < 100 ppm > 100 ppm Impurity Considerations
Optical Spectometry – Absorption
IR Air B •24 AI 0.0UZ AI 0.0Z Polyatomic and Heteroatomic
(Infrared) Ar B •26 AR 5.0UH AR 5.0UH Compounds
Oxygen can oxidize a sample. Moisture
N 2 B •65 NI 5.0UH NI 5.0UH interferes with IR spectra. Impurities
LN 2 B •85 Liquid Liquid coinciding with analyte peaks can cause
inaccuracies.
AA Air B •24 AI 0.0Z AI 0.0Z Elemental Analysis
(Atomic Absorption) Ar B •26 AR 5.0UH AR 5.0UH Impurities can cause the flame to discolor
or burn unevenly. Furnace atmospheres
C 2 H 2 B •23 AC 2.6AA AC 2.6AA require low oxygen and moisture levels to
n-C 4 H 10 B •31 BU 2.5IS BU 2.5IS maintain instrument sensitivity.
H 2 B •54 HY 4.5Z HY 4.5Z
N 2 B •65 NI 5.0UH NI 5.0UH
N 2 O B •69 NS 2.5AA NS 2.5AA
Gas Grade Legend Please refer to designated page numbers for available
AA – Atomic Absorption cylinder styles, contents, and other information.
EC – Electron Capture Detector Please refer to the Instrumentation Mixture Summary Laboratories
IS – Instrument on page D •218 for additional information regarding mixtures.
RS – Research/Chromatographic Please refer to the Regulator Reference Guide on page E •241
TG – Trace Analytical for help in selecting the appropriate cylinder regulator.
UH – Ultra High Purity
UZ – Ultra Zero
Z – Zero
Application Note:
Helium and Hydrogen Carrier Gases for Gas Chromatography
Helium and hydrogen are the most common carrier gases another can involve significant changes to standard operat-
used for gas chromatography applications. These two gases ing procedures. In executing a conversion from helium to
deliver similar optimum achievable separation efficiencies hydrogen, one must consider not only compatibility of the
(theoretical plates). Hydrogen offers advantages versus gas chromatograph but also the associated plumbing and
helium in having a higher and broader range of linear connected components (e.g., filters or mass spectrometer).
velocities for which near-optimum separation efficiency can Hydrogen presents flammability and asphyxiation hazards,
be achieved, especially for applications requiring longer and appropriate safety precautions should be taken in
columns (higher inlet pressures). The higher linear velocities accordance with careful risk assessment when working with
available to hydrogen enable significant potential reduc- hydrogen in a lab. Helium is typically supplied via cylinders,
tions in analysis time. Unlike inert helium, hydrogen has the while hydrogen can be supplied via cylinders or on-site
potential to react with some analytes; such reactions are generators. With decades of experience in supplying gases
uncommon under normal gas chromatography conditions. for analytical instruments, Praxair is prepared to help users
Users should note that conversion from one carrier gas to identify and move forward their preferred best gas solutions
for their particular applications.
1-877-PRAXAIR www.praxairdirect.com D•215